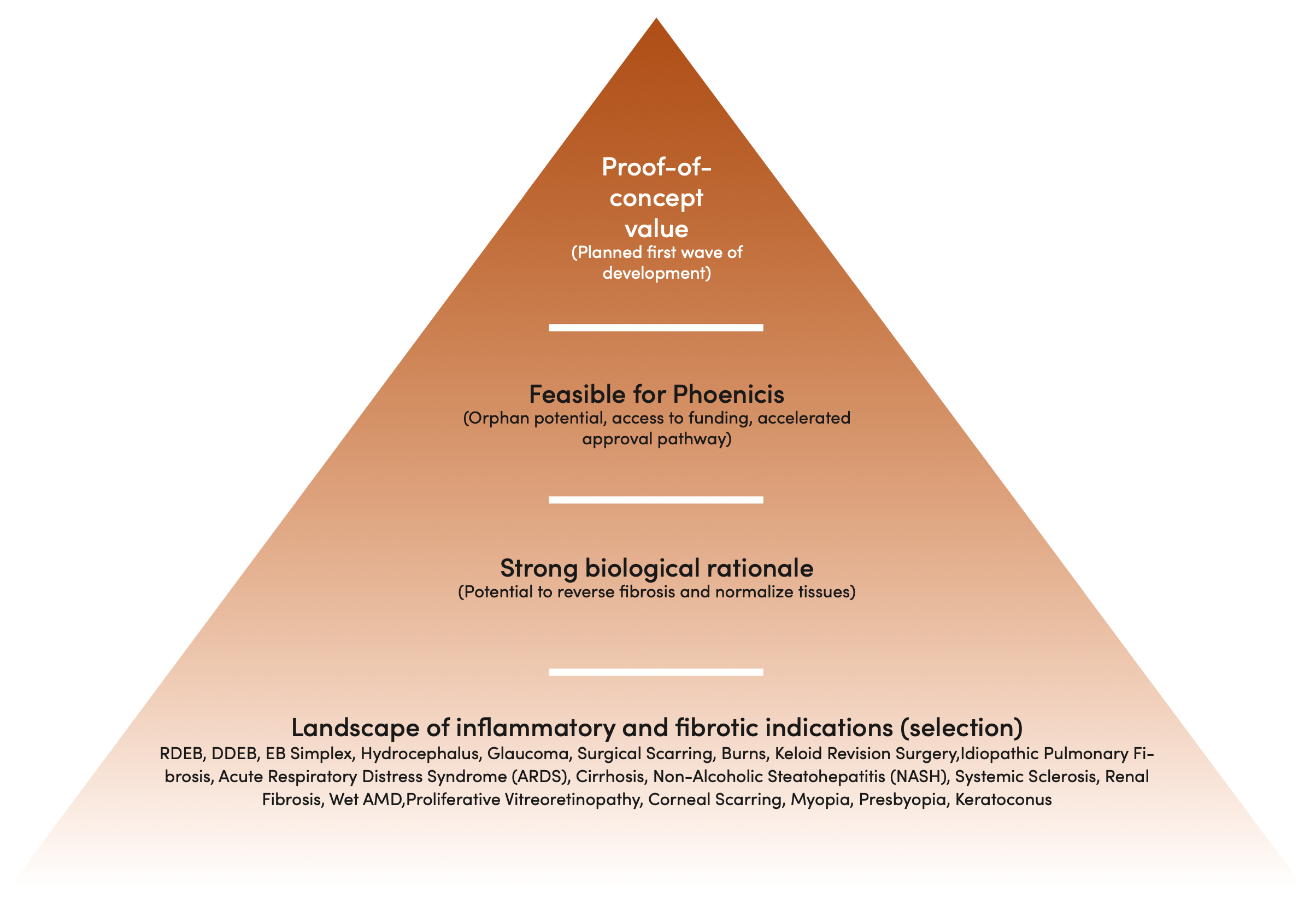
We are committed to improving the lives of those who suffer from rare disorders. Our long track record of value creation and a mission-driven mindset has strong KOL support. Our R&D team has created 15+ businesses, advanced 12+ drugs into the clinic, and has been involved with 4 FDA and 3 EMA drug approvals in the last decade.
Our therapy in IND enabling studies for Epidermolysis bullosa and will be ready for the clinic mid-2024.